The full HPS mortality data have never been properly published and one of the HPS authors just asked me WHY I wanted to know, instead of coming up with the data. One of the letter authors in Lancet Aug. 30 [who also was not given an answer] figures they won't ever provide these data --and that should be denounced in the Journals [project... U/C/M/P?].
I'm not a skeptic but there is nothing simpler than those morality data. NNT/y to postpone 1 death in HPS is ~300 [at the precise moment HPS chose to report final mortality, if that helps - but what if it was 6 months earlier? Nobody knows].
I attach the PDF of the letters in Lancet Aug. 30, unsuccessfully asking for complete mortality data.
Only 2 of the last 4 big trials give cumulative mortality data [see link below] but PROSPER claims no mortality benefit, yet HPS DOES. HPS may well have been stopped at a coincidentally particularly advantageous moment in the statistical ups and downs [as ASCOT was stopped with a small NS benefit showing, while 3 months before the curves touched].
The refusal to provide such vital prescribing data is immoral but that's big pharma, I'm afraid, but why Oxford U.? Think EXCEL: stopped at 11 months with a 275% [still just NS] increased rate of deaths in lovastatin [pharma owned the study and the authors have never published anything in Medline since [that I could find], so they cannot be corresponded with -and a ~20% sub section that was continued was never reported on.].
P.S. page 747 of that Lancet attachment has WHO calling a Pfizer consumer ad cam
paign in France "unethical";
that's the campaign we're trying to stop in Canada
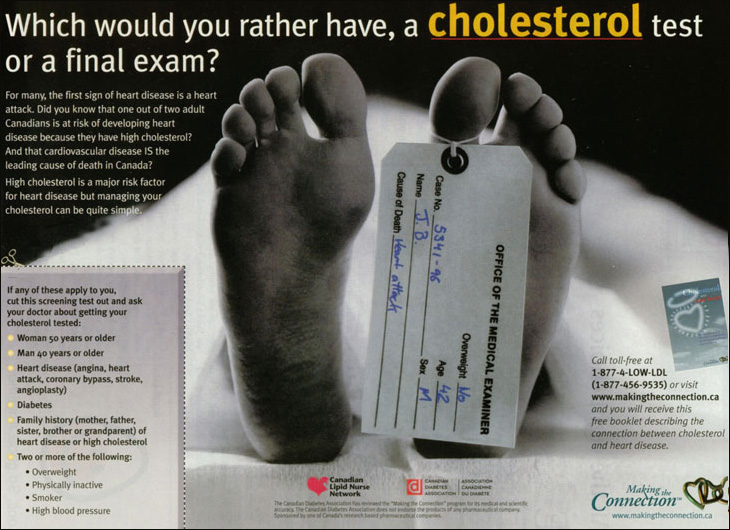
as denounced
http://www.cmaj.ca/cgi/eletters/169/5/425
The HPS study paper is available for free, probably thanks to Merck.
Cheers, Eddie