26
____________________________________________________________
POLLUTION AND SMOKER'S SCURVY
Great effort is being expended to cleanse our environment and there is hope for eventual success in minimizing man-made contamination. There are, however, some areas which may not fully respond, and a complete elimination of pollutants certainly is not likely in the foreseeable future. One area of pollution is the natural background radiation level due to cosmic and other radiation from the sun and outer space. Even when crowds of people congregate they are irradiating themselves and surrounding people with the rays from the radioactive potassium in their bodies. Another segment is in carbon monoxide exposure. While a major source of local carbon monoxide buildup in the atmosphere is due to combustion of fuels, (estimated to produce 200 million tons a year), even if this were completely eliminated, there would still be other sources. Carbon monoxide is produced naturally in the human body a the rate of 0.42 milliliter per hour and a major source of carbon monoxide intoxication is cigarette smoking (1). The oceans are a natural source for carbon monoxide (2) as well as plants (1). The air over the oceans and virgin forests, far from human contamination, would thus never be free of a low, but definite, level of carbon monoxide. Traces of carbon monoxide are even found in stored soft drinks (3). Thus, it would be impossible for one to avoid carbon monoxide altogether.
Measures to lessen direct contamination of the air and soil are being developed, but it may take years for them to take noticeable effect. In the meantime, a supplemental approach to this problem, which is being suggested is to make the population more resistant to the harmful effects of the pollutants by using ascorbic acid. The previous chapters on the effects of ascorbic acid in combating the various chemical and physical stresses suggest a valid basis for this new approach. The daily administration of a few grams of ascorbic acid may be adequate to increase the resistance of the body to these chronic toxic environmental stresses.
In the case of carbon monoxide toxicity, a 1962 paper from the Soviet Union (3) showed that the chronic exposure of guinea pigs to carbon monoxide increased the rate of consumption of an requirement for, ascorbic acid. The effects of chronic carbon monoxide poisoning were counteracted by administering 40 milligrams of ascorbic acid daily. For a 300-gram guinea pig, 40 milligrams are equivalent to 9,000 milligrams (9 grams) for a 150-pound body weight. In 1955, Klenner (3) noted that the treatment of choice for carbon monoxide poisoning, both acute and chronic, was ascorbic acid. Two other papers need to be mentioned in this discussion, one appearing as far back as 1930 and the other in 1958. Ungar and Bolgert (4) showed that ascorbic acid would protect guinea pigs against death from exposure to high concentrations of hydrochloric acid vapor, nitric oxide, and other vapors. To be effective, however, it was necessary to give not less than 500 milligrams per kilogram of body weight, which is equivalent to about 35,000 milligrams for a 150-pound body weight. Ozone, a necessary constituent of the upper atmosphere and a contaminant produced in the air we breathe under certain conditions, is a toxic oxidizing substance. Mittler (4), in 1958, reported that a single injection of ascorbic acid into mice before exposing them, for 3 hours, to air containing an ozone level of 8 to 25 parts per million, provided a higher rate of survival than among untreated mice.
Will the agencies now so concerned with our external environment also be concerned with our individual internal environment and implement research on the use of ascorbic acid to combat environmental hazards?
Smoking
Smoking is an intense form of concentrated individual air pollution. With all the furor about cleaning up the air we breathe, smokers nonchalantly inhale the concentrated smoke from a plug of burning tobacco an inch or two from their face. This smoke contains levels of gaseous pollutants -- carbon monoxide, hydrocyanic acid, nitric oxide, sulfur dioxide, and acetonitrile -- in much higher concentrations than would ever be permitted in the air we breathe. In addition, the smoke contains finely dispersed carcinogenic tars, poisons such as nicotine, radioactive dust such as polonium-210, and other ingredients. These are deposited on the tissues of the mouth, tongue, pharynx, bronchi, and interior of the nose. We have here a highly irritating form of local chemical stress which depletes the tissues of their stores of ascorbic acid. Research may eventually show that this chronic irritation and depletion are what finally trigger the neoplastic process.
Many reports can be found in the medical literature on the destructive influence of tobacco smoke on the ascorbic acid levels of the body. As far back as 1939, Strauss and Scheer (5) found that smoking produced a constant and marked reduction in the excretion of ascorbic acid, indicating its destruction in the body by smoke constituents. McCormick (5), in 1952, stated:
In determining the anti-infectious protective dosage of vitamin C there is another factor which is not generally considered. When the vitamin is employed to neutralize toxins of endogenous or exogenous origin, the action is reciprocal in that the vitamin is also neutralized proportionally, leaving less available for physiological needs. To illustrate, the writer has determined by laboratory and clinical tests that the smoking of one cigarette neutralizes in the body approximately 25 milligrams of vitamin C, or the amount in one medium-sized orange. It will thus be seen how difficult it is to meet the bodily requirement of the pack-a-day smoker for even the protective level of vitamin C from dietary sources. It is thus obvious that the steady smoker, who is usually short on his dietary intake as well, requires a much heavier therapeutic dosage of this vitamin than the nonsmoker.
Bourquin and musmanno (5), in 1953, reported that nicotine, added to human blood, decreased its ascorbic acid content by 24 to 31 percent. They concluded that larger amounts of ascorbic acid should be taken by habitually heavy smokers.
Venulet, in a series of papers published during 1951 to 1956, as reviewed by Andrzejewski (5), showed that the inhalation of tobacco smoke produced a marked loss of ascorbic acid in animals and man. In 60 medical students, the blood ascorbic acid levels were 1.0 to 1.2 mg % in nonsmokers, and 0.6 to 0.9 mg % in smokers. The nonsmokers who volunteered to smoke six to eight cigarettes per day suffered a significant decrease in serum ascorbic acid levels by the third day. This drop disappeared five days after cessation of the smoking. If the levels in man parallel those found in animals,there is a large decrease for each internal organ. There are considerable differences in the ascorbic acid content found in the milk of smoking and nonsmoking mothers. Maximal differences occur in the spring when the levels average 5.9 mg % in nonsmoking women against 2.1 mg % in smoking women. Venulet concluded that smoking is involved in the pathogenesis of certain widespread disorders such as scurvy, gastric ulcer, and cardiovascular disease. Apart from difficulties arising from ascorbic acid depletion, smoking lowers the general bodily resistance to disease.
Goyanna (5), in 1955, in a paper entitled "Tobacco and Vitamin C" thoroughly indicts tobacco smoking for its destructive action on the body's ascorbic acid. He showed that in heavy smokers, ascorbic acid is destroyed and no longer excreted in the urine. He described the many functions of ascorbic acid in the body including its role in detoxicating poisons. In his concluding paragraph he states, "The salvation of the smoker may be in this vitamin."
In 1960, Dietrich nd Büchner (5). demonstrated lowered blood plasma ascorbic acid levels in smokers and concluded that smokers exhibit a vitamin C deficiency compared to nonsmokers. They advised all smokers to consume an abundance of vitamin C to prevent development of a deficiency.
Durand et al. (5), in 1962, also confirmed that the blood ascorbic acid of smokers is lower than nonsmokers. He gave 1 gram of ascorbic acid a day to pack-and-a-half cigarette smokers and found the increases in blood ascorbic acid never attained the peak levels shown by nonsmokers. He concluded that there is an ascorbic acid deficiency in smokers. Rupniewska (5), in 1965, also reported a decreased store of ascorbic acid in aged smokers.
Calder, Curtis and Fore (5), in 1963, indicated that tobacco smoke destroyed vitamin C in solution. They also demonstrated a statistically significant difference in blood plasma ascorbic acid and leukocyte ascorbic acid contents of cigarette smokers and non-smokers. The more cigarettes smoked, the lower were the ascorbic acid blood levels.
A 1968 study by Brook and Grimshaw (5) demonstrated that blood plasma and leukocyte ascorbic acid levels are significantly lower in men than in women. In nonsmokers, the plasma levels declined with age while the leukocyte levels did not. Cigarette smoking was found to significantly lower both the blood plasma and the leukocyte ascorbic acid concentrations. Heavy smoking had the same effect on the blood plasma ascorbic acid as increasing the chronological age by 40 years.
Pelletier (5), in tests on five smokers and five nonsmokers, as reported in 1968, demonstrated that the ascorbic acid levels of the blood and blood plasmas of smokers were about 40 percent that of nonsmokers. On giving his subjects 2 grams of ascorbic acid a day he found that after continued administration the blood ascorbic acid levels stabilized at approximately the same value for both groups. However, the urinary excretion of ascorbic acid by the smokers never reached the levels excreted by the nonsmokers, which indicated a continuing greater utilization of the ascorbic acid by the smokers. He also make tests on guinea pigs which were fed nicotine for one month in amounts comparable to those consumed by heavy smokers. There was a drop in the blood and tissue ascorbic acid, as compared to guinea pigs fed the same diet without the nicotine, amounting to 49 percent in the adrenal gland, 50 percent in the kidneys, 47 percent in the heart, and 34 percent in the liver.
From all this research work, it should be evident that habitual smokers, unless they take steps to correct the condition, are likely to be in a chronic, subclinical scorbutic state. In this situation, the classical signs of scurvy may not be manifest, but the body is in a state of biochemical scurvy. With this depletion, there is lowered resistance to disease and the biochemical detoxication processes are impaired. I have termed this bodily condition, "Smoker's Scurvy" and a very simple cure for it is -- stop smoking.
For the numerous hard-core smokers who cannot kick the habit, cigarette manufacturers could institute a program of research to determine if the long-term daily use of ascorbic acid will provide some protection against cancers, emphysema, coronaries, and other diseases which afflict smokers.
Research conducted at Tulane University by Schlegel and coworkers (6), and mentioned previously, prompted Schlegel to recommend the daily use of 1.5 grams of ascorbic acid to prevent the recurrence of bladder cancer in smokers.
27
____________________________________________________________
WOUNDS, BONE FRACTURES, AND SHOCK
It has been known for hundreds of years that wounds will not heal and that healed old wounds and scars reopen in people deprived of ascorbic acid and afflicted with scurvy. Scurvy also weakens the bones and renders them more susceptible to fracture. In the forty years since the discovery of ascorbic acid, there have been so many papers published on the beneficial relationship of ascorbic acid to wound healing, on the improved strength of the scar tissue, and on the faster healing of bone fractures, that it is just impossible to fully review this vast volume of work within a brief chapter. However, the interested reader can refer to the papers presented at the Scientific Conference on vitamin C, held by the New York Academy of Sciences in 1960 (1). The papers by Abt and Schuching, Robertson, Gould, Crandon, Fullmer, and Lee are of particular interest in this connection.
The utilization of ascorbic acid in wound healing is now so well documented that there are many surgeons who routinely provide their patients with 1 or 2 grams of ascorbic acid a day, postoperatively, to aid in their healing and recovery. In spite of the vast number of published papers, it is still not known whether these are the optimal levels for this purpose and whether the patients would benefit from the administration of higher amounts. This should be the subject of further research.
Of particular interest at this point is the recent work of Dr. Steinberg (1), of Jewish Memorial Hospital in New York City, in the successful treatment of gangrene of the legs and feet with sodium ascorbate. In five cases of long-standing gangrene, resistant to other forms of treatment and some scheduled for amputation, the administration of up to 5 grams of sodium ascorbate daily, in addition to other treatment brought about improvement and healing in a few weeks. These cases of gangrene were caused by arteriosclerotic occlusion, diabetic endarteritis, and polycythemia vera. While the ascorbic acid status of the patients was not determined, it is likely they were suffering from severe chronic subclinical scurvy. Much follow-up work is needed on this promising lead in the treatment of gangrenous lesions.
But even in wound healing, the full potential of ascorbic acid may not be entirely exploited. For instance, there is a critical shortage of hospital beds and all the medical and other facilities necessary to maintain them. Research should be instituted to determine if the daily routine administration of a few grams of ascorbic acid to hospital admissions would hasten their recovery and shorten their hospital stay. Many patients now entering hospitals are already in a prescorbutic state. If their hospital stay could be shortened by 25 percent, it would be equivalent to building and staffing a new facility for every four in existence, at only pennies a day per patient.
Morbidity Index -- A new Diagnostic Tool
Another idea of medical treatment which requires more investigation is the use of ascorbic acid as a diagnostic and prognostic tool. Blood samples are usually taken from patients and a variety of examinations are made on them. It is rare, however, that a determination of ascorbic acid is ever made on these blood samples. Because of complications in the methodology developed over the years, the determination of ascorbic acid in blood has lost much of its diagnostic value and has fallen into disrepute.
The methods in use during the 1930s determined the true, "reduced" ascorbic acid in the blood. In 1943, when new procedures were introduced, what was determined was the "total" ascorbic acid, which not only included the "reduced" ascorbic acid but the "oxidized" dehydroascorbic acid and other decomposition products. The actual results obtained by these two different types of methods were not comparable and caused much confusion, which still exists. While it is possible to separately determine the ascorbic acid and the dehydroascorbic acid be either techniques, it was seldom carried out and reported in the research work of the past forty years.
The development of a possible valuable diagnostic
tool was delayed for four decades due to a lack of appreciation of the simple
physicochemical facts involved. What is needed in these determinations is
not the "total" or the "reduced" ascorbic acid, but
the ratio of the two components, ascorbic acid and dehydroascorbic
acid. In 1955, Chakrabaryi and Banerjee (2), after reviews of the prior
work, pointed out the paradox of dehydroascorbic acid which, at low levels,
behaves essentially like ascorbic acid in giving protection from or curing
scurvy, but is toxic at high levels. They determined both the ascorbic
acid and dehydroascorbic acid in the blood of many of their patients.
They found that the ascorbic acid levels went down and dehydroascorbic acid
levels went up as their patients became sicker and finally died from
meningitis, tetanus, pneumonia, and typhoid fever. If the patients
survived, the trend was reversed. Hoffer and Osmond (2), in 1963, cited
many other references relating to mental stress and mental disease affecting
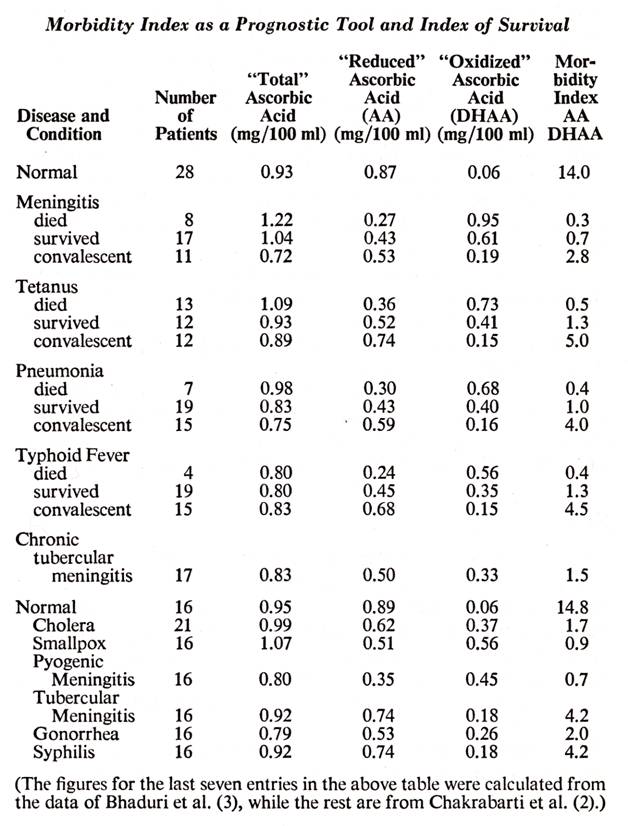
the ascorbic acid blood levels and also first calculated the ![]() ratios which showed some startling
statistics. These figures, along with some others which I calculated from
another paper (3), are assembled in the following table.
ratios which showed some startling
statistics. These figures, along with some others which I calculated from
another paper (3), are assembled in the following table.
Inspection of the figures on this table shows the
inadequacy of "total" ascorbic acid blood levels as a diagnostic
measurement. Because of the high dehydroascorbic levels, many of the dea
patients had higher "total" levels than the survivors. With
many investigators during the past three decades reporting "total"
ascorbic acid in their work, it can easily be discerned how the present confusion
and lack of confidence in blood ascorbic determinations resulted. The
"reduced" ascorbic acid levels are a superior indicator, but most
significant is the ratio of ![]() which I term the "morbidity index".
which I term the "morbidity index".
The "normals" had a morbidity index of approximately 15 although an individual taking high levels of ascorbic acid would have even a higher index. Those who were critically sick bur survived had a morbidity index of about 1.0, while those who died had much less, 0.3, to 0.5. During convalescence of the survivors, the morbidity index jumped to 3.0 to 5.0
There is a logical physicochemical explanation for these variations. Ascorbic acid and dehydroascorbic acid, as explained in earlier chapters, are members of a reversible oxidation-reduction system. The redox potential depends on the relative amounts of each component of the systems. For healthy tissue processes, that ratio must favor high amounts of ascorbic acid and very low levels of dehydroascorbic acid in order to keep the redox potential low. In pathology, the tissue potentials approach more oxidative levels as the disease progresses, and recedes again as the disease clears up.
Incalculable time has been wasted by hundreds of investigators over the past forty years of research work. They were trying to relate ascorbic acid blood levels to a disease process, but did not realize these simple facts and only determined and reported either "total" ascorbic acid or "reduced" ascorbic acid. Much of the confusion during the past four decades is due to this inadequate data.
The value of megascorbic therapy may be in maintaining the redox tissue potentials at the necessary low levels and maintaining the morbidity index in the upper brackets. The constant presence of high ascorbic acid levels may suppress the formation of the toxic dehydroascorbic acid.
Here we have in a potentially valuable tool which may aid the physician in determining how sick his patient really is and his chances for survival. Further research should resolve the question of how valuable a tool the morbidity index really can be.
Shock
Shock is a very dangerous condition of general bodily collapse that can rapidly appear as a result of the stresses of severe, traumatic injuries, burns, major surgery, massive hemorrhage, abdominal injury, and dehydration. The fundamental defect in shock is failure of effective blood flow and hence impaired transport of vital materials in the blood to the organs and tissues. This is brought on by increased permeability of the capillaries, resulting in loss of blood plasma into the surrounding tissues. The lowered blood volume, with its increased percentage of blood cellular constituents, is more difficult to pump through the arteries and veins. The volume of blood being pumped from the heart is low and the blood pressure is low. The patient is usually in a state of collapse with a pallid, moist skin and impaired mental faculties. It is necessary to rapidly correct this condition and usually the first measures are to assure proper breathing and replace the blood's volume of fluid.
The use of ascorbic acid in the treatment of shock has been repeatedly suggested in many papers over the past thirty years. These papers include reports not only of successful experiments on laboratory animals, but on case histories on man. There is a perfectly good rationale for this use of ascorbic acid because of its long-known beneficial effects in preventing capillary fragility and the fact that hemorrhage is a characteristic symptom of ascorbic acid depletion. In 1941, in experiments on cats bled of 50 percent of their blood volume, Stewart and coworkers (4) were able to prolong the animals' lives with an intravenous injection of ascorbic acid. Ungar (4), in 1942 and 1943, was able to prevent traumatic shock and death in injured guinea pigs by an injection of ascorbic acid of 100 milligrams or more per kilogram of body weight. In 1943, nearly three decades ago, he noted that particular emphasis should be laid on the possibility of utilizing his observations in the treatment of human traumatic and surgical shock.
Further tests on guinea pigs, reported in 1944 by McDevitt and coworkers (4), showed ascorbic acid increased their resistance to trauma and improved their survival. In studies on eleven human subjects undergoing major surgery they sampled the patients' blood for ascorbic acid determinations before, during, and after the operations. Of the eleven cases, five had subnormal ascorbic acid levels before the operation and eight had levels markedly below normal immediately postoperatively or within twenty-four hours.
Hemorrhagic shock induced in guinea pigs by a standardized bleeding procedure was the subject of a 1946 paper by de Pasqualini (4). She found that if the guinea pigs were given 200 milligrams of ascorbic acid five minutes before the start of bleeding, it prevented shock and 94 percent of her eighteen test animals survived, while 90 percent of the seventeen animals not given ascorbic acid died.
Holmes (5), in 1946, discussed the use of ascorbic acid to relieve the increased capillary permeability occurring in shock. He cites the successful results of a group of cooperating surgeons using ascorbic acid to successfully treat surgical shock. Another surgeon used it successfully preoperatively and postoperatively in fifty serious abdominal operations. In approximately 2,000 cases of dental extractions, ascorbic acid was administered thirty to forty-five minutes before the extraction, preventing shock and postoperative weakness. It was also employed in thirty-five cases of mine injuries, in which instances it helped the injured to survive the long trip to the hospital. He also cites the experience of other cooperating physicians. He noted that there was no question on the value of adequate amounts of ascorbic acid in the maintenance of a healthy condition of the capillary walls and that it may also be useful in combating the anoxia of shock. The highest blood levels of ascorbic acid were reached after two or three hours on oral administration and in three to five minutes when given intravenously.
In 1946, S.M. Leverson and associates (6), reported on the use of ascorbic acid, vitamins B1, and B2, and nicotinic acid in severe injury, hemorrhage , and infections in humans. They concluded that their work adds further support to the idea that large doses of these materials may serve a useful purpose in treating acutely ill patients. In 1962, a conference and workshop on hemorrhagic shock was held at the Rockerfeller Institute. As reported in Science by Simeone (6), Dr. Levenson presented a paper revealing that injured animals suffer for biochemical scurvy. Levenson's claim that ascorbic acid could influence the mortality from hemorrhagic shock was "viewed with skepticism."
In 1947, Zerbine (7), reported on a duodenal ulcer operation where the patient went into postoperative shock. Prompt administration of 2 grams of ascorbic acid, intravenously, pulled him out of the shock within minutes. Continued large dosages of ascorbic acid made the patient's recovery "uneventful." Zerbini noted that his one observation is merely suggestive but that "further studies would obviously seem worthwhile." In the course of tests in eighty surgical operations, Pataky and associates (7) reported in 1957 that they were able to inhibit the passage of plasma through the intact vessel walls, and to control surgical shock with large doses of ascorbic acid. Kashchevskaia (7), in a 1958 paper from the Soviet Union, showed that ascorbic acid is intimately involved in the state of shock.
Strawitz and coworkers (8), using rats to determine the effect of ascorbic acid and methylene blue in hemorrhagic shock, reported in 1958 that both agents significantly reduced the mortality rates and, in addition, ascorbic acid lengthened the survival period.
In 1963, Santome' and Gomez (9), using dogs as experimental animals, checked the earlier work of Sayers et al. on rats in hemorrhagic shock. They found an increase in the blood ascorbic acid levels and a highly significant decrease in the blood ascorbic acid levels and a highly significant decrease in the adrenal gland ascorbic acid, in spite of the higher levels in the blood. The higher blood level is likely to be an artifact because the liver rapidly synthesized ascorbic acid under the stress and it poured it into a reduced volume of blood. The low ascorbic acid levels in the adrenal glands are probably a more reliable criteria of the response to the heavy stresses to which the animals were subjected.
In a paper published in 1967, Kocsard-Varo (9) discussed microcirculation (the capillary system), capillary permeability, and ascorbic acid. She made the following interesting observations which involved her in the study of the microcirculation. She found that in nosebleed due to high blood pressure, if a 1-1,000 adrenaline solution were applied to the surface of the nasal mucous membrane or if ascorbic acid were injected individually, the bleeding would continue. However, if they were both done simultaneously, the blood flow stopped "instantaneously, as if one turned off a faucet. The bleeding does not recur." Ascorbic acid has a known protective action on adrenaline in the circulation.
The electron microscope studies of the capillary bed of ascorbic acid-deficient guinea pigs, by Gore and coworkers (10), published in 1968, disclosed the ultrastructural basis for the capillary defects, the microdiscontinuities and microlesions which lead to capillary fragility.
The above review of the highly suggestive research over the past three decades illustrates the possible usefulness of ascorbic acid in the prevention and treatment of traumatic, hemorrhagic, and surgical shock. Yet how much use is being made of this data in present-day shock therapy? In a paper published in 1969 by Weil and Shubin (11), workers in the Shock Research Unit of a large medical school and hospital, there is not a single mention of ascorbic acid.
With shock still claiming so many victims in highway accidents and battlefield casualties, the need for more work in this area is urgent.
28
____________________________________________________________
Pregnancy, parturition, and lactation are periods of intense biochemical stress for mammals. Pregnant mammals, such as rats, which have the ability to produce their own ascorbic acid, significantly increase production of this metabolite to combat these stresses. The recommended daily dietary allowance of ascorbic acid for pregnant and lactating women as set forth by the Food and Nutrition Board of the National Academy of Sciences, is 60 milligrams a day (1). Their corresponding recommendations for nonpregnant females, aged eighteen to over seventy-five, is 55 milligrams a day. This provides a meager 5 milligrams a day to maintain homeostasis under the biochemical stresses of a developing baby, the labor of childbirth, the production of milk, and the physiological recovery from the rigors of motherhood itself.
Let us make a simple calculation, assuming that a lactating mother produces 500 to 1,000 milliliters of breast milk daily (about a pint to a quart). This is the amount a normal infant should consume in the first three months of life according to Ingalls (1). The milk should contain more than 4 milligrams of ascorbic acid per 100 milliliters, according to Snelling (1). This adds up to an additional burden on the mother of 20 to 40 milligrams of ascorbic acid a day which is secreted for the nourishment of the baby. If the lactating mother is only getting a total of 60 milligrams of ascorbic acid a day, this extra load leaves her only 20 to 40 milligrams of ascorbic acid daily for her own hard-working physiology -- 15 to 35 milligrams a day less than is recommended for a nonlactating female. Draw your own conclusions.
That ascorbic acid is of vital importance in the biochemistry of pregnancy and fetal development can be seen from the profound effects on animals deprived of ascorbic acid. As far back as 1915, before the discovery of ascorbic acid, it was known that the stresses of pregnancy made guinea pigs more susceptible to scurvy. When pregnant guinea pigs were placed on a scorbutic diet early in pregnancy, it led to abortion or absorption of the fetuses, while ascorbic acid deprivation in the latter half of pregnancy resulted in stillbirths or delivery of premature or weak, scorbutic young. Female guinea pigs on a scorbutic diet did not become pregnant and there were profound changes in their ovaries, as described by Kramer and coworkers in 1933. If fed inadequate levels of orange juice, pregnant guinea pigs either aborted or resorbed the fetuses or failed to give birth to living young, depending upon when the experimental animals were deprived of ascorbic acid and the extent of deprivation. Previously, in 930, it had been shown by Goettsch that depriving guinea pigs of vitamin C not only interfered with the estrus cycle of the females, but the males lost their ability to sire litters. In her paper, Goettsch quoted earlier work, going back to 1919, which showed the deleterious effect of scurvy on the sexual activity of guinea pigs (2).
It is interesting to note in this connection that a simple test, proposed in 1968 by Paeschke and Vasterling (3) to determine the time of ovulation is based on variations of ascorbic acid in the urine. A marked decrease in urinary ascorbic acid signals the time of ovulation. A similar test was proposed as far back as 1940 by Pillary. Ascorbic acid is also important in the ripening of the human egg, as shown by the 1963 studies of Bertetti and Nonnis-Marzano. This paper contains a bibliography with eighty-five references (3).
As cited in the 1958 paper of Räihä, in a study of over 2,000 women, Martin found an increased frequency of premature births in mothers with the lowest intake levels and lowest serum concentrations of ascorbic acid. In over 200,000 deliveries in Finland, the frequency of stillbirths was highest in December and January and lowest in September, Dietary intakes of ascorbic acid are generally higher in the summer and early fall when fresh fruits and vegetables are available, and they are lowest in the winter months. Pankamaa and Räihä also cited the 1955 paper by Sauvage Nolting, who reported on defective brain development in human subjects caused by ascorbic acid deficiency (4).
In general, it appears that the mother herself suffers more from a diet low in ascorbic acid than the fetus. There seems to be a selective transfer of ascorbic acid by the placenta from the mother's blood to the fetus, as observed by McDevitt in 1942 (5). The ascorbic acid content of the blood of the fetus at birth is higher than the mother's (Manahan and Eastman, 1938; Mindlin, 1949; and Slobody et al. 1945) and the fetus seems to act parasitically (Teel 1938) tending to deplete the mother's supply when her intakes are low. In spite of this, the fetus still may not obtain enough ascorbic acid and congenital scurvy can occur, as shown by Jackson and Park in 1035 (5) and Ingier (2) in 1915.
There is a considerable body of medical literature from 1937 to 1964 indicating that ascorbic acid deficiencies and deprivation are intimately involved with spontaneous abortion, habitual abortion, and premature rupture of the fetal membranes. Therapy which included the use of ascorbic acid proved effective in correcting these conditions (6).
Mammals that produce their own ascorbic acid may not always produce enough to completely overcome the stresses of reproduction and can benefit from additional amounts. It has been reported by Phillips et al. (7), in 1941, that injections of ascorbic acid in "hard to settle" cows resulted in 60 percent of them becoming pregnant on breeding. Similarly, ascorbic acid injections improved the condition of a large percentage of sterile and partially sterile bulls.
Thus, there seems to be general agreement that ascorbic acid deficiency, due to low intakes by the mother during pregnancy, can have serious consequences in the reproductive process. It was only when laboratory animal tests were conducted to determine the effect of added ascorbic acid that contradictory results were obtained which will require further clarification. A program of long-term definitive research work is needed to determine the optimal level of intake for pregnancy, for easing the stresses of parturition and labor, and for the postnatal care of the mother and child.
The following is a brief review of the contradictory data from tests on guinea pigs. In the 1962 publication of the National Research Council of the National Academy of Sciences, "Nutrient Requirements of Laboratory Animals," two diets are given as satisfactory for raising guinea pigs, and hundreds of generations of guinea pigs have probably been raised using them One diet supplies 12.5 milligrams of ascorbic acid per day, while the other supplies 50 milligrams of ascorbic acid daily. Assuming the guinea pig weighs about 300 grams, these amounts are equivalent to 2.9 grams and 11.7 grams of ascorbic acid respectively for a 70-kilogram body weight (154 pounds).
In 1951, a paper by Dr. W. Neuweiler (8) appeared that reported on tests with pregnant guinea pigs which were given 25 milligrams of ascorbic acid daily in addition to their vegetable diet. He stated that, in spite of the fact that there were no general toxic manifestations, there were disturbances to the reproductive process, with fertility changes and increased fetal mortality. He did not mention the number of guinea pigs used in his tests. In 1953, Mouriquand and Edel (8) reported using 250 milligrams of ascorbic acid daily by injection or ingestion (ten times more than Neuweiler used and equivalent to 117 grams of ascorbic acid on a 70-kilogram body weight basis). Males an nonpregnant female guinea pigs were unaffected, but for 3 pregnant females there were shortened gestation periods with increased stillbirths. On the other hand, Lamden and Schweiker (9), in 1955, gave daily intraperitoneal injections of 100 to 200 milligrams of ascorbic acid for six weeks (about 23 grams to 47 grams of ascorbic acid daily for a 70-kilogram body weight) and reported, "there was no interference in the gestation and bearing of healthy litters by two guinea pigs injected with the above amounts of ascorbic acid for a major part of the gestation period."
M.L. Steel 99), in 1968, in a Ph.D. thesis entitled "Growth and Reproduction of Guinea Pigs Fed Three Levels of Ascorbic Acid," used in her work 4, 10, and 100 milligrams per kilogram of body weight (equivalent respectively to 280 milligrams, 700 milligrams, and 7.0 grams per 70-kilogram adult body weight). She showed that animals on the lowest level of ascorbic acid intake had more difficulty in starting a pregnancy, the pregnancy was more likely to end unsuccessfully, the abortion rate was higher, and the death rate was higher. The highest level of ascorbic acid appeared to protect the parent animal from obvious malfunction of the reproductive faculty. However, the survival rate of the offspring was highest when the parent animal was fed the lowest level.
This latter result requires further investigation because it contradicts the results reported in 1967 by C.G. King (9), who supplemented the diet of female guinea pigs each day with 1.5, 3, 6, and 20 milligrams of ascorbic acid. Growth rates were comparable in all groups, but the number of viable offspring increased with each increase in dosage. Survival records were the lowest and stillbirths and resorptions were the highest for the group receiving the least ascorbic acid.
The Soviet worker E.P. Samborskaia (10), in 1962, tested the effect of ascorbic acid on the reproduction system of guinea pigs and mice and reported changes in the organs and sexual cycles of the animals. According to Steel (9), Samborskaia introduced the ascorbic acid intravaginally into the animals by means of cotton-wool tampons soaked in an ascorbic acid solution, a highly unusual means of application.
In 1964, Samborskaia reported on tests on pregnant guinea pigs given 50 to 500 milligrams of ascorbic acid each day (about 12 to 120 grams per 70 kilograms of body weight), stating that there were increases in abortions, stillbirths, and births of nonviable young. In her 1966 paper, she reported the administration of 150 milligrams of ascorbic acid daily (about 35 grams per 70 kilograms of body weight) to 14 pregnant rats. Three of these rats aborted on the thirteenth to fifteenth day of pregnancy. She also reports tests on women which translate as follows:
There were twenty women, ages twenty to forty, who had come to the gynecologist with the request for an abortion. Sixteen of the twenty women under observation began to menstruate one to three days after receiving the prescribed course of ascorbic acid. There was no effect on four of the women.
The "prescribed course" appears to have been 6 grams of ascorbic every twenty-four hours for a three-day period. She concluded that this increases the levels of estrogens, which in turn serves to provoke abortion. Besides language difficulties, many Soviet medical papers have a frustrating lack of essential details, but if her results are reliable, then there are wide implications for the use of ascorbic acid in this field, especially since the legalization of abortions. However, many other workers have used ascorbic acid for just the opposite purpose in the treatment of threatened abortion and habitual abortion: Pearse and Trisler (10), in 1957; Ainslee (10), in 1959; and also the numerous workers reporting in the papers cited under reference (6). It is difficult to reconcile the views of this one Soviet worker with the results reported by others.
Samborskaia's work on abortion seems particularly suspicious when viewed in the light of Klenner's results (11), reported in 1971, on megascorbic prophylaxis in over 300 human pregnancies. His patients were given orally, throughout their pregnancies, from 4 grams to 15 grams of ascorbic acid a day on approximately the following schedule; 4 grams daily in the first trimester, 6 grams daily in the second trimester, and 10 grams daily during the last trimester. Approximately 20 percent in this series required 15 grams ascorbic acid a day in the lst trimester. There were no miscarriages in the entire series, and one woman in the series had ten consecutive normal pregnancies and ten healthy babies. On admission to the hospital for childbirth, 80 percent of the patients were given a booster injection of 10 grams of ascorbic acid intravenously. Labor was shorter, less painful, and uncomplicated. Striae gravidarum (abdominal wrinkles after childbirth) was seldom seen and there were no postpartum hemorrhages. During childbirth, the perineum was remarkably elastic and episiotomy was performed electively. Healing was always by first intention. Fifteen to twenty years after the last childbirth, the firmness of the perineum is found to be like that during the first childbirth, provided the patient continued on large daily intakes of ascorbic acid. No toxic manifestations were demonstrated in this series and there was no cardiac stress even though twenty-two patients in the series had "rheumatic hearts."
The most remarkable effects of the megascorbic dosages were the health and vigor of the babies. They were all robust and not one required resuscitative measures. All the babies from the series were so strong, good looking, vigorous, and trouble-free that the nurses in the hospital referred to them as the "Vitamin C Babies." The Fultz quadruplets were in this series and they are the only quadruplets that have survived in southeastern United States. The babies were given 50 milligrams of ascorbic acid on the first day and dose was gradually increased thereafter until they were taking 1 gram a day at one year of age. This recommended routine daily dose is increased 1 gram for each year until ten years and then 10 grams regularly thereafter. This megascorbic regimen in pregnancy and childbirth certainly deserves wider recognition and use.
For the induction of labor in childbirth, the administration of ascorbic acid was proposed by Spitzer (12), in 1947; and Tasch (12), in 1951, used it to shorten the period of labor and to favorably influence the postlabor period. McCormick (12), in 1948, suggested ascorbic acid as a means of avoiding the striae of pregnancy (striae gravidarum or striae atrophicae). Further research would also be profitable in these areas and also in the treatment of painful menstruation (13), excessive menstrual bleeding (14), and in relief of menopausal disorders (15).
29
____________________________________________________________
One area where considerable research with ascorbic acid has been expended is in the treatment of schizophrenia. A substantial amount of clinical information has been collected in the chemotherapy of this disease using the megascorbic and megavitamin approach. In a 1967 report (1) of twelve independent psychiatric studies, 80 percent of 1,500 schizophrenics, continuously treated with megascorbic and megavitamin therapy, showed recovery or marked improvement. It was estimated that, 1,500 doctors in the United States and Canada and over a hundred institutions were using this treatment.
At the present time this research and therapy are continuing at a rapid pace and a publicly supported foundation, the American Schizophrenia Foundation, not only has been involved in much of this research, but also has plans for organizing a research and demonstration facility, which will eventually carry a patient load of 3,000 to 5,000 annually. Biochemical investigations of mental diseases will be conducted in this facility which will also act as a training facility for doctors. Further information is available form this foundation and from its chapters in many states, Canada, and Bolivia.
In 1884, J.W.L. Thudichum (2), who is regarded by many as the father of modern neurochemistry, published a book on brain chemistry in which he advanced the hypothesis that "many forms of insanity" are caused by "poisons fermented within the body," that is, toxic substances produced or delivered to the brain by a faulty metabolism. He also suggested that these unknown processes would become quite obvious when we acquired a better understanding of the biochemistry of the brain. He spent the next ten years in the isolation and characterization of some of the chemical constituents of the brain. However, it is only the research of the past several decades that has confirmed this keen foresight.
The discovery and synthesis of ascorbic acid initiated such a large volume of research that, in 1938, Wacholder compiled a review to determine the extent of the interest of this work for neurology and psychiatry. In 1940, Lucksch reported his results in his paper, "Vitamin C and Schizophrenia." Also in 1940, Soloveva, from the Soviet Union, reported favorable results from its use in various psychoses. Berkenau, in 1940, suggested that the delay in the ascorbic acid "saturation" of his small group of psychotic patients may be significant. In 1951, another review by Low-Maus, "Vitamin C and the Nervous System," appeared with seventy-six references (3).
In the period from 1953 to 1955, De Sauvage Nolting (4) published a series of short papers on the relation of ascorbic acid to mental disease. Form 1957 to 1966, there appeared papers published in various parts of the world which showed that mental disease patients have high demands for ascorbic acid, have subnormal body levels, and, in many cases, are in a state of subclinical scurvy. Several of the papers suggested that mental patients should be given large doses of ascorbic acid (5).
VanderKamp (6), in 1966, found that schizophrenics metabolized ascorbic acid at rate 10 times that of a control group. He gave 6 to 8 grams of ascorbic acid every 4 hours to a group of ten schizophrenics, or a total of 36 to 48 grams of ascorbic acid a day. All ten of the patients showed definite clinical improvement.
This review indicates the practical usefulness of massive doses of ascorbic acid in the treatment of schizophrenia, but it does not give us much information as to how it works or any insight as to what are Thudichum's "poisons fermented within the body" which may cause mental illness.
In the case of schizophrenia, a brilliant series of research papers, started in the early 1950s by A. Hoffer, H. Osmond, and their coworkers (7) has brought much light into this are and has been a major factor in confirming Thudichum's insight. It has also served to return the biochemistry of the body to psychiatry after a long attempt to exile it in favor of culture and psyche. This research was based on a hypothesis which resulted from two simple observations (8):
1. There is chemical similarity between adrenalin, the normal secretion of the adrenal gland, and mescaline, a hallucinatory drug.
2. The psychological effects of mescaline on humans in many ways resemble the symptoms of acute schizophrenia. The faulty metabolism of adrenalin in the body of a schizophrenic, possibly due to a genetic defect (9), could lead to an adrenalin metabolite with psychological properties resembling those of mescaline, but much more potent. If such a substance were made in the body, then clinical schizophrenia would result.
It was suggested that the faulty metabolism of adrenalin led to an increased production of adrenochrome, which they showed was an hallucinogen. Their hypothesis suggested that the adrenochrome was partially responsible for the changes produced in schizophrenia, and it would follow that any method for decreasing adrenochrome production from adrenalin would be therapeutic for schizophrenia. This was attempted by using massive doses of the vitamin, niacin, to reduce the formation of the precursor, adrenalin, from noradrenalin. Clinical experience has been good and has shown the value of including high levels of the other B vitamins in the therapy. Ascorbic acid is also used at levels of 1 to 6 grams a day and the reasons for using ascorbic acid were the subject of a comprehensive paper by Hoffer and Osmond (5). Their research has been long and continuing and its reception has been stormy, which is the usual course for a new concept. The final test is in the clinical results, which appear good.
Linus Pauling (10), in a 1967 paper entitled, "Orthomolecular Somatic and Psychiatric Medicine," proposed a new means of treating disease. The therapy merely provides the optimal molecular constitution of the body, especially the optimal concentration of substances which are normally present in the human body and are required for life itself. It is known that the proper functioning of the mind requires the presence in the brain of molecules of many different substances, such as the B vitamins and ascorbic acid, and malfunction may result if optimal levels are not maintained. This work, which provides the rationale for the use of large doses of normal metabolites in the treatment of mental disease, was further elaborated in a paper on "Orthomolecular Psychiatry" in 1968. In a talk presented at the Second International Conference of Social Psychiatry in London in 1969, Pauling suggested that an optimal intake of ascorbic acid could mean a 10 percent improvement in physical and mental health. He speculated, "What would be the consequences for the world if the national leaders and the people as a whole were to think just 10 percent more clearly?"
The effect of continued mega levels of ascorbic acid on human intelligence is a subject which has never been examined. Teets could easily be applied on two populations of children from similar backgrounds and economic levels. One group would be maintained on their present intake levels of ascorbic acid from their foodstuffs, which inadequately corrects their hypoascorbemia, and the other group continuously maintained (from birth, if possible) on the levels, suggested by Dr.F.R. Klenner, of 1 gram ascorbic acid per day, per year of age, up to age ten, and then 10 grams a day thereafter. Some startling improvements on intelligence levels may be observed by this orthomolecular approach.
As in other chapters, this review must be brief and incomplete. Because of limited space, many important references containing significant contributions to this work have been omitted. If the reader is interested, reference may be made to a recent more detailed review (11) which cites 191 references.
Happily, in the concluding chapter, some organized research can be cited in megascorbic and megavitamin therapy which has yielded definite benefits. It is hoped that this is only the beginning of a trend in organized and coordinated research in megascorbic therapy.
30
____________________________________________________________
The large amount of medical literature cited in Chapters 12 to 29 represents but a small fraction of the total work published during the last forty years on the use of ascorbic acid for diseases other than scurvy. This vast volume of research time and energy was expended by hundreds of workers scattered all over the world with a complete lack of coordination of their experimental techniques, purpose and background. Their total effort has settled few problems of practical importance and has resulted, at best, in many suggestions for future research. The bulk of this uncoordinated effort has produced a gigantic waste of time and money and a confusing mass of conflicting results and opinions. While these four decades of work have established the dosage of ascorbic acid necessary to prevent frank clinical scurvy, we still are not sure of such simple facts as the optimal intakes of ascorbic acid needed for good health and resistance to disease processes, and how dosage requirements vary from individual to individual and under stress. Past research, because of its haphazard nature, lack of coordinated effort, and inability to carry an investigation to its ultimate final solution, has created more problems than it has solved. Much greater progress should have been made in these last forty years than has actually occurred.
To avoid these pitfalls in future research, and because of the broad range of potential uses of ascorbic acid, the establishment of a central agency, to coordinate, initiate, sponsor, and direct the future research effort is required. Such an agency would also act as a clearinghouse for pertinent information and regularly publish, at frequent intervals, the results of the current research. Most important is the staffing of that agency. It should be a multidisciplinary group of unbiased, imaginative scientists with a wide background on the modern concepts of ascorbic acid; it should comprise clinicians, M.D.'s, biochemists, and scientists from other disciplines to tackle every possible problem.
The National Institutes of Health in Bethesda, Maryland, could set up and organize a new division, 'Hypoascorbemia and Mega-ascorbic Medicine," to function in this capacity. If Congress could be convinced of the urgent need for this concentrated effort and its probable beneficial effects on American public health, it could direct the Department of Health, Education, and Welfare to accomplish this. It is the fond hope of the author that, as further research is conducted on hypoascorbemia and the realization of its importance is confirmed, a National Megascorbic Authority will eventually be organized with general aims and purposes similar to the proposed National Cancer Authority.
No claim is made for the complete coverage of the subject matter of Chapters 12 to 19. Other areas of investigation would be part of the program of the agency, such as verifying the reports of the beneficial action of ascorbic acid in multiple sclerosis, Menier's syndrome, hemophilia, and insomnia, to name a few. The megavitamin therapy of schizophrenia is now actively being investigated by the publicly supported American Schizophrenia Association and the Huxley Institute for Biosocial Research. These new agencies could cooperate and work closely with those foundations.
The main purpose of this agency would be to determine the optimal levels of ascorbic acid intakes based on the genetic concepts, under "normal" and stressed conditions, and the human individual variations and responses, Such an agency could determine the safety of megascorbic prophylaxis and megascorbic therapy, and make available practical applications of these measures with the shortest time lag. Presently, we can only entertain a hope that future generations may live longer and healthier lives because of these ideas.
Another factor which has impeded the development of the wider usage of ascorbic acid in megascorbic prophylaxis and megascorbic therapy has been a lack of convenient dosage forms for these purposes. For oral administration, the largest easily available tablet contains only 500 milligrams and the largest chewable tablet has 250 milligrams of ascorbic acid. If one wants to consume 10 grams of ascorbic acid a day, it is necessary to swallow twenty large tablets or eat forty chewables. The less readably available powdered ascorbic acid or sodium ascorbate, when measured by the teaspoonful (a level teaspoonful is about 3 grams) and dissolved in water or fruit juice, is convenient and palatable when obtainable. This also avoids the possibility of discomfort when swallowing a series of large tablets. What is really needed for oral administration is a pleasantly flavored chewable wafer supplying 2 or 3 grams of ascorbic acid.
The situation is even worse for doctors who want to use megascorbic therapy by injection. The only injectable ascorbic acid now available in the trade comes in small ampuls containing, at most, 1 gram of ascorbic acid. If a doctor wants to administer a therapeutic injection of 30 or 40 grams, he must break open and combine the contents of at least thirty or forty small glass ampuls in order to obtain the required dosage. Only an unusually dedicated physician will go to this trouble. What is needed here is a wide distribution of large ampuls with sterile solutions containing 20 to 40 grams of sodium ascorbate suitable for injection. Similarly, for longer-term parenteral fluid therapy by the intravenous route, bottles of parenteral solutions containing up to 30 grams of sodium ascorbate per liter are needed. The only product distributed at present is a vitamin mixture containing, at most, about a gram of vitamin C per liter. The availability of these products would make megascorbic therapy, for the practicing physician in his office an hospital, a practical reality for both emergencies and routine therapy.